INTRODUCTION
The existence of micro emulsions was first described by Schulman in (1943)and Winsor (1954) and the definition of micro emulsion described by Lindman is as “micro emulsion is a system of water oil an empty file which has a single optically isotropic and thermodynamically stable liquid solution”.1-3 However, the history of discovery of Micro emulsion was well before the study of Schulman and the first commercial micro emulsion, liquid boxes was discovered in 1928 and later it gained significant attention in the oil industry.4 Nowadays it has potential application in separation science, environmental science ,materials science and reaction engineering with certain unique advantages.5
can be used as a Nano reactor to form a mono dispersed nanoparticles of drug delivery vehicle due to the fast exchange between the droplets in micro emulsions. 6-7
showing serious adverse effects. This is a significant step forward in the delivery of poorly soluble drugs. Micro emulsion systems are also now being increasingly investigated for transdermal, ocular, nasal, pulmonary, vaginal, rectal and intravenous drug delivery. 8 Micro emulsions are generally classified as water in oil, oil in water or bi-continuous system depending on surfactant type, sample environment etc and they are characterized by ultra-low interfacial tension between oil and water phases. In water in oil in micro emulsion, the surfactant rich oil phase coexist with the surfactant pour acquiesce face and in oil in water micro emulsion, the surfactant rich water phase coexist with the oil phase where surfactant is only present as monomer at small concentration micro emulsion, which contain water or highly polar solvents like methanol(CHOH) acetone nitrile(ACN), dimethyl form amide (DMF) in the core, have been studied extensively.9-16 The size of the micro emulsion is varied in the order of nanometer and it is generally characterized by the R/W value (R/W=[water or polar solvents]/[surfactants]). Recently, several attempts have been made for the preparation of micro emulsion where various polar solvents with high dielectric constant and which are miscible in nonpolar solvents are used instead of water in polar core.17-19 These micro emulsions are important in their article as well as experimental point of view and recently they are widely applied to semiconductors, solar energy conversion, micro colloids etc.20-21 Moreover these waterless micro emulsions have significant advantages over water-based micro emulsions in different organic reactions such as Diels -Alder reaction, esterification, polymerization etc.22 Recently room temperature ionic liquids received an increasing number of attention is because of their unique physio-chemical properties such as low volatility, high thermal stability and high ionic conductivity and the drawbacks associated with the non-aqueous solvent or organic solvents related to environment, health or safety can be conquered by using the room temperature ionic liquids.23-28 More importantly, the cationic and anionic constituents of the room temperature ionic liquids can be modified to obtain the desired property of the solvent and therefore they are termed as designer solvent. For this they are frequently used in organic synthesis, catalyses, electrochemical studies and other chemical and technological applications. 29-33 IL exist as liquid in room temperature because of their chemical structure. The cations and anions of the ILs are chosen in such a way that they destabilize the solid face Crystal and they are stabilized by the strong electrostatic interaction and other weak non-specific interaction between them.
At first, researchers were attempting to solubilize different ionic liquids replacing water from the core of the micro emulsion.34-35 Later it was observed that IL can also play the role of organic solvent and from the green chemistry point of you it is very much promising for applications. Besides this water and oil can also solubilize in oil-based surfactant.
DEFINITION: Micro emulsion is defined “as system of water, oil, and amphiphile which is optically isotropic and thermodynamically stable liquid solution”.
IUPAC DEFINITION: Micro-emulsion- Dispersion made of water, oil, and surfactant(s) that is an isotropic and thermodynamically stable system with dispersed domain diameter varying approximately from 1 to 100 nm, usually 10 to 50 nm.
STRUCTURE OF MICROEMULSION:

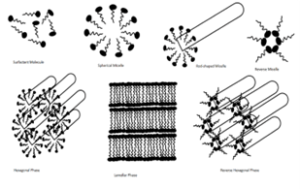
Micro emulsions are dynamic systems in which the interface is continuously and spontaneously fluctuating.38 Structurally, they are divided into oil-in- water (o/w), water in-oil (w/o) and bi continuous micro emulsions. In w/o micro emulsion, water droplets are dispersed in the continuous oil phase while o/w micro emulsion is formed when oil droplets are dispersed in the continuous aqueous phase. In systems where the amounts of water and oil are similar, a bi continuous micro emulsion may result. In all three types of micro emulsions, the interface is stabilized by an appropriate combination of surfactants and/or co-surfactants. The mixture of oil, water and surfactants is able to form a wide variety of structures and phases depending upon the proportions of the components. The flexibility of the surfactant film is an important factor in this regard. A flexible surfactant film will enable the existence of several different structures like droplet like shapes, aggregates and bi continuous structures, and therefore broaden the range of micro emulsion existence. The micro emulsion structure is shown in Fig. 1.The schematic representation given in Fig. 2 gives an indication of a few of the wide variety of possible self-association structures that surfactants can form in the presence of water, oil or combinations of all three. The possible structural representations of the three different types of micro emulsions which are most probably likely to be formed depending on their individual composition. It can be seen that while the oil-in-water (o/w) and water-in-oil composition (w/o) droplets are represented as spheres, they may be also be asymmetric in shape, frequently looking like the shape of a prolate ellipsoid.
The likely presence of o/w micro emulsion droplets is a feature in micro emulsions where the volume fraction of oil is low. Conversely, w/o droplets are likely when the volume fraction of water is low and in systems where the amount of water and oil are similar, a bi continuous micro emulsion may result.
COMPONENTS OF MICROEMULSION
- Oil Phase
- Surfactant
- Co-surfactant
OIL PHASE: The oil represents one of the most important excipients in the formulation not only because it can solubilized the required dose of the lipophilic drug, it can increase the fraction of lipophilic drug transported via the intestinal lymphatic system, thereby increasing absorption from the GI tract depending on the molecular nature of the triglyceride.39 The oil component influences curvature by its ability to penetrate and hence swell the tail group region of the surfactant monolayer. Short chain oils penetrate the tail group region to a greater extent than long chain alkanes, and hence swell this region to a greater extent, resulting in increased negative curvature. Saturated (for example, lauric, myristic and capric acid) and unsaturated fatty acids (for example, oleic acid, linoleic acid and linolenic acid) have penetration enhancing property of their own and they have been studied since a long time.
SURFACTANTS: The surfactant chosen must be able to lower the interfacial tension to a very small value which facilitates dispersion process during the preparation of the microemulsion and provide a flexible film that can readily deform around the droplets and be of the appropriate lipophilic character to provide the correct curvature at the interfacial region.40 Surfactants used to stabilize microemulsion system may be: i.Non-ionic, ii. Zwitterionic, iii. Cationic, or iv. Anionic surfactants. It is generally accepted that low HLB (3-6) surfactants are favored for the formulation of w/o microemulsion, whereas surfactants with high HLB (8-18) are preferred for the formation of o/w microemulsion. Surfactants having HLB >20 often require the presence of co-surfactants to reduce their effective HLB to a value within the range required for microemulsion formation.
CO-SURFACTANT: In most cases, single-chain surfactants alone are unable to reduce the o/w interfacial tension sufficiently to enable a microemulsion to form. The presence of co-surfactants allows the interfacial film sufficient flexibility to take up different curvatures required to form microemulsion over a wide range of composition. If a single surfactant film is desired, the lipophilic chains of the surfactant should be sufficiently short, or contain fluidizing groups (e.g. unsaturated bonds). Short to medium chain length alcohols (C3-C8) are commonly added as co surfactants which further reduce the interfacial tension and increase the fluidity of the interface. Typical co-surfactants are short chain alcohols (ethanol to butanol), glycols such as propylene glycol (PG), medium chain alcohols, amines or acids.
FACTORS AFFECTING THE FORMATION OF MICROEMULSION: The formation of oil or water swollen micro emulsion depends on the packing ratio, property of surfactant, oil phase, temperature, the chain length, type and nature of co-surfactant.41-42
METHOD OF PREPARATION OF MICROEMULSION:
4.1. PHASE TITRATION METHOD: Microemulsions are prepared by spontaneous emulsification method (phase titration method) and can be depicted with the help of phase diagrams. Construction of phase diagram is a useful approach to study the complex series of interactions that can occur when different components are mixed. Microemulsions are formed along with various association structures (including emulsion, micelles, lamellar, hexagonal, cubic, and various gels and oily dispersion) depending on the chemical composition and concentration of each component. The understanding of their phase equilibria and demarcation of the phase boundaries are essential aspects of the study. As quaternary phase diagram (four component system) is time consuming and difficult to interpret, pseudo ternary phase diagram is often constructed to find the different zones including microemulsion zone, in which each corner of the diagram represents 100% of the particular component.
PHASE INVERSION METHOD: Phase inversion of microemulsions occurs upon addition of excess of the dispersed phase or in response to temperature. During phase inversion drastic physical changes occur including changes in particle size that can affect drug release both in vivo and in vitro. These methods make use of changing the spontaneous curvature of the surfactant. For non-ionic surfactants, this can be achieved by changing the temperature of the system, forcing a transition from an o/w microemulsion at low temperatures to a w/o microemulsion at higher temperatures (transitional phase inversion).
5. CONSTRUCTION OF PSEUDO TERNARY PHASE DIAGRAM:

When water, oil and surfactants are mixed, microemulsion is only one of the association structures. Preparation of a stable, isotropic homogeneous, transparent, non-toxic microemulsion requires consideration of a number of variables. Construction of phase diagrams reduces a number of trials and labor. Phase diagrams help to find the microemulsion region in ternary or quaternary system and also help to determine the minimum amount of surfactant for microemulsion formation. Pseudo-ternary phase diagrams of oil, water, and co- surfactant/ surfactants mixtures are constructed at fixed cosurfactant/surfactant weight ratios. Phase diagrams are obtained by mixing of the ingredients, which shall be pre-weighed into glass vials and titrated with water and stirred well at room temperature. Formation of monophasic/biphasic system is confirmed by visual inspection. In case turbidity appears followed by a phase separation, the samples shall be considered as biphasic. In case monophasic, clear and transparent mixtures are visualized after stirring the samples shall be marked as points in the phase diagram. The area covered by these points is considered as the microemulsion region of existence. Fig 3. shows hypothetical pseudo- ternary diagram at constant surfactant to co-surfactant ratio. It also shows that single phase or multiphase regions of microemulsion domains are near the center of diagram in areas containing large amounts of surfactant that is toxic. The phase behavior of surfactants, which form microemulsion in absence of co-surfactant, can be completely represented by ternary diagram.43
TYPES OF MICROEMULSION SYSTEMS: According to Winsor there are 4 types:

WINSOR I: The microemulsion composition corresponding to Winsor I is characterized by two phase, the lower oil/water (O/W) microemulsion phase in equilibrium with excess oil.
WINSOR II: The microemulsion composition corresponding to Winsor II is characterized by very low interfacial tension and maximal solubilization of oil and water for a given quantity of surfactant. Since, in this phase, microemulsion coexists with both excess phases, no one can distinguish the dispersed phase from the continuous phase.
WINSOR III: This phase comprises of three phases, middle microemulsion phase (O/W plus W/ O, called bicontinuous) in equilibrium with upper excess oil and lower water.
WINSOR IV: Microemulsions can be distinguished from the micelles by its inner core swollen with oil. The microemulsion structure depends on the chemical composition, temperature and concentration of the constituents.
APPLICATIONS OF MICROEMULSIONS:
It can be used as a delivery system by the various routes as below:
- Oral Delivery: Eg. – Piroxicam
- Parenteral Delivery :Eg. -Flurbiprofen
- Topical Delivery: Eg. -Dexamethasone
- Ophthalmic Delivery: Eg. -Chloramphenicol
- Nasal Delivery: Eg. – Sumatriptan
- Drug Targeting
- Brain Targeting
- Periodontal Delivery
- Cellular Delivery
ADVANTAGES OF MICROEMULSION:
- Microemulsion acts as super solvent for drug.
- Ability to carry both hydrophilic and lipophilic drug.
- Due to small droplet size it has large interfacial area of globule so drug is rapidly released in external phase when absorption takes place.
DISADVANTAGES OF MICROEMULSIONS:
Require large amount of surfactant and co-surfactant.
- Limited solubility for high melting substances.
- Stability influenced by environmental parameters such as temperature and pH
DIFFERENT ROLE OF IL IN THE
FORMULATION OF MICROEMULSION
Based on the role of ILs, microemulsions can be classified into three categories:
- Non aqueous IL microemulsions: The polar domain of the microemulsion constitutes the IL.
- Aqueous IL microemulsion: The nonpolar part constituents the IL and water is used in polar domain.
- Microemulsions with IL as surfactants: Different Surface Active Ionic Liquids (SAIL) are used as surfactant and in the polar domain aqueous as well as non aqueous solvents including IL are used.
EVALUATION OF MICROEMULSION:
| Parameters | Technique Used |
| Phase Behaviour | Phase contrast microscopy & freeze fracture TEM |
| Size and Shape | Transmission Electro Microscopy(TEM) |
| Rheology | Brookfield Viscometer |
| Conductivity | Conductivity Meter |
| Zeta Potential | Zetasizer |
| pH | pH Meter |
| Drug Release Studies | Frans Diffusion Cells |
CONCLUSION
The use of microemulsions as drug delivery vehicle has been an exciting and attractive area of research because of its any potential and extraordinary benefits. In microemulsions, one can design the interface of such nanometer sized droplets so that droplet stability and lifespan in humans can be made to last from a few milliseconds to minutes, or even to hours. The interfacial rigidity of the microemulsion droplets plays a key role in the flux of the drugs from such droplets to the cells and Tailoring of microemulsion systems to control the flux of the drugs can be done so as to customize drug delivery according to individual patient requirements or to specific pharmaceutical needs. Microemulsions offer an interesting and potentially quite powerful alternative carrier system for drug delivery because of their high solubilization capacity, transparency, thermodynamic stability, ease of preparation, and high diffusion and absorption rates when compared to solvent without the surfactant system. In last few decades ionic liquids (ILs) have been widely considered as a green solvent and they are used in various fields. ILs can be used in the formation of micro emulsion as a dispersed medium, polar domain and recently as a surfactant. ILs with a certain surface activity having long alcohol chain substitutes can self-aggregate and form Ionic Liquid Micro Emulsion with high-temperature insensitivity.
REFERENCE
- Schulman, J. H., Stoeckenius, W., & Prince, L. M. (1959). Mechanism of formation and structure of micro emulsions by electron microscopy. The Journal of physical chemistry, 63(10), 1677-1680.
- Winsor, P. A. (1948). Hydrotropy, solubilisation and related emulsification processes. Transactions of the Faraday Society, 44, 376-398.
- Danielsson, I. (1981). The definition of microemulsion.
- Kunz, W., Zemb, T., & Harrar, A. (2012). Using ionic liquids to formulate microemulsions: current state of affairs. Current opinion in colloid & interface science, 17(4), 205-211.
- Greaves, T. L., & Drummond, C. J. (2008). Ionic liquids as amphiphile self-assembly media. Chemical Society Reviews, 37(8), 1709-1726.
- Sarkhejiya Naimish, A., Nakum Mayur, A., Patel Vipul, P., Atara Samir, A., & Desai Thusarbindu, R. (2000). Emerging trend of microemulsion in formulation and research. International bulletin of drug research, 1(1), 54-83.
- Beckman, E. J. (1996). Carbon dioxide extraction of biomolecules. Science, 271(5249), 613-613.
- Challa, V., Kuta, K., Lopina, S., Cheung, H. M., & von Meerwall, E. (2003). Microporosity of bicontinuous nanoporous polymeric materials, characterized with restricted diffusion. Langmuir, 19(10), 4154-4161.
- Venables, D. S., Huang, K., & Schmuttenmaer, C. A. (2001). Effect of reverse micelle size on the librational band of confined water and methanol. The Journal of Physical Chemistry B, 105(38), 9132-9138.
- Boyd, J. E., Briskman, A., Sayes, C. M., Mittleman, D., & Colvin, V. (2002). Terahertz vibrational modes of inverse micelles. The Journal of Physical Chemistry B, 106(24), 6346-6353.
- Pileni, M. P., Zemb, T., & Petit, C. (1985). Solubilization by reverse micelles: solute localization and structure perturbation. Chemical Physics Letters, 118(4), 414-420.
- Pileni, M. P., Brochette, P., Hickel, B., & Lerebours, B. (1984). Hydrated electrons in reverse micelles: 2 quenching of hydrated electron by sodium nitrate. Journal of colloid and interface science, 98(2), 549-554.
- Kahlweit, M., Strey, R., & Busse, G. (1990). Microemulsions: a qualitative thermodynamic approach. Journal of Physical Chemistry, 94(10), 3881-3894.
- Pileni, M. P. (1993). Reverse micelles as microreactors. The Journal of physical chemistry, 97(27), 6961-6973.
- Li, N., Gao, Y. A., Zheng, L., Zhang, J., Yu, L., & Li, X. (2007). Studies on the micropolarities of bmimBF4/TX-100/toluene ionic liquid microemulsions and their behaviors characterized by UV− Visible spectroscopy. Langmuir, 23(3), 1091-1097.
- Gao, Y. A., Li, N., Zheng, L., Bai, X., Yu, L., Zhao, X., … & Li, Z. (2007). Role of solubilized water in the reverse ionic liquid microemulsion of 1-butyl-3-methylimidazolium tetrafluoroborate/TX-100/benzene. The Journal of Physical Chemistry B, 111(10), 2506-2513.
- Correa, N. M., Silber, J. J., Riter, R. E., & Levinger, N. E. (2012). Nonaqueous polar solvents in reverse micelle systems. Chemical Reviews, 112(8), 4569-4602.
- Falcone, R. D., Correa, N. M., Biasutti, M. A., & Silber, J. J. (2000). Properties of AOT aqueous and nonaqueous microemulsions sensed by optical molecular probes. Langmuir, 16(7), 3070-3076.
- López-Cornejo, P., & Costa, S. M. (1998). Luminescence of zinc tetraphenylporphyrin in ethylene glycol-in-oil microemulsions. Langmuir, 14(8), 2042-2049.
- Schubert, K. V., Lusvardi, K. M., & Kaler, E. W. (1996). Polymerization in nonaqueous microemulsions. Colloid and Polymer Science, 274(9), 875-883.
- Zein El Abedin, S., & Endres, F. (2007). Ionic liquids: the link to high-temperature molten salts?. Accounts of chemical research, 40(11), 1106-1113.
- Dupont, J. (2011). From molten salts to ionic liquids: a “nano” journey. Accounts of chemical research, 44(11), 1223-1231.
- Rogers, R. D., & Seddon, K. R. (2003). Ionic liquids–solvents of the future?. Science, 302(5646), 792-793.
- Greaves, T. L., & Drummond, C. J. (2008). Protic ionic liquids: properties and applications. Chemical reviews, 108(1), 206-237.
- Castner Jr, E. W., & Wishart, J. F. (2010). Spotlight on ionic liquids. The Journal of chemical physics, 132(12), 120901.
- Hayes, R., Warr, G. G., & Atkin, R. (2015). Structure and nanostructure in ionic liquids. Chemical reviews, 115(13), 6357-6426.
- Welton, T. (1999). Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chemical reviews, 99(8), 2071-2084.
- Podolean, I., Hardacre, C., Goodrich, P., Brun, N., Backov, R., Coman, S. M., & Parvulescu, V. I. (2013). Chiral supported ionic liquid phase (CSILP) catalysts for greener asymmetric hydrogenation processes. Catalysis today, 200, 63-73.
- Bouvy, C., Baker, G. A., Yin, H., & Dai, S. (2010). Growth of gold nanosheets and nanopolyhedra in pyrrolidinium-based ionic liquids: investigation of the cation effect on the resulting morphologies. Crystal growth & design, 10(3), 1319-1322.
- Tsuda, T., & Hussey, C. L. (2007). Electrochemical applications of room-temperature ionic liquids. The electrochemical society interface, 16(1), 42.
- Bhattacharyya, K. (2010). Room-temperature ionic liquid: a nanostructured liquid for high-vacuum and high-energy applications. The Journal of Physical Chemistry Letters, 1(21), 3254-3255.
- Kuchlyan, J. and Kundu, N., 2016. Ionic liquids in microemulsions: formulation and characterization.Current Opinion in Colloid & Interface Science, 25, pp.27-38.
- Hoar, T. P., & Schulman, J. H. (1943). Transparent water-in-oil dispersions: the oleopathic hydro-micelle. Nature, 152(3847), 102-103.
- Frenkel, M., Chirico, R. D., Diky, V., Brown, P. L., Dymond, J. H., Goldberg, R. N., … & Williams, P. A. (2011). Extension of ThermoML: The IUPAC standard for thermodynamic data communications (IUPAC Recommendations 2011). Pure and Applied Chemistry, 83(10), 1937-1969.
- Kimura, M., Shizuki, M., Miyoshi, K., Sakai, T., Hidaka, H., Takamura, H., & Matoba, T. (1994). Relationship between the molecular structures and emulsification properties of edible oils. Bioscience, biotechnology, and biochemistry, 58(7), 1258-1261.
- Strickley, R. G. (2004). Solubilizing excipients in oral and injectable formulations. Pharmaceutical research, 21(2), 201-230.
- Kumar, R. S., & Mukherjee, J. (2019). Microemulsions: platform for improvement of solubility and dissolution of poorly soluble drugs. Journal of Drug Delivery and Therapeutics, 9(4-A), 908-910.
- Lam, A. C., & Schechter, R. S. (1987). The theory of diffusion in microemulsion. Journal of colloid and interface science, 120(1), 56-63.
- Shafiq-un-Nabi, S., Shakeel, F., Talegaonkar, S., Ali, J., Baboota, S., Ahuja, A., … & Ali, M. (2007). Formulation development and optimization using nanoemulsion technique: a technical note. AAPS pharmscitech, 8(2), E12-E17.
- Ho, H. O., Hsiao, C. C., & Sheu, M. T. (1996). Preparation of microemulsions using polyglycerol fatty acid esters as surfactant for the delivery of protein drugs. Journal of pharmaceutical sciences, 85(2), 138-143.
- Dreher, F., Walde, P., Walther, P., & Wehrli, E. (1997). Interaction of a lecithin microemulsion gel with human stratum corneum and its effect on transdermal transport. Journal of controlled release, 45(2), 131-140.
- Lv, F. F., Li, N., Zheng, L. Q., & Tung, C. H. (2006). Studies on the stability of the chloramphenicol in the microemulsion free of alcohols. European journal of pharmaceutics and biopharmaceutics, 62(3), 288-294.
- Li, L., Nandi, I., & Kim, K. H. (2002). Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. International journal of pharmaceutics, 237(1-2), 77-85.

